 Pharmacovigilance Course
Pharmacovigilance Course
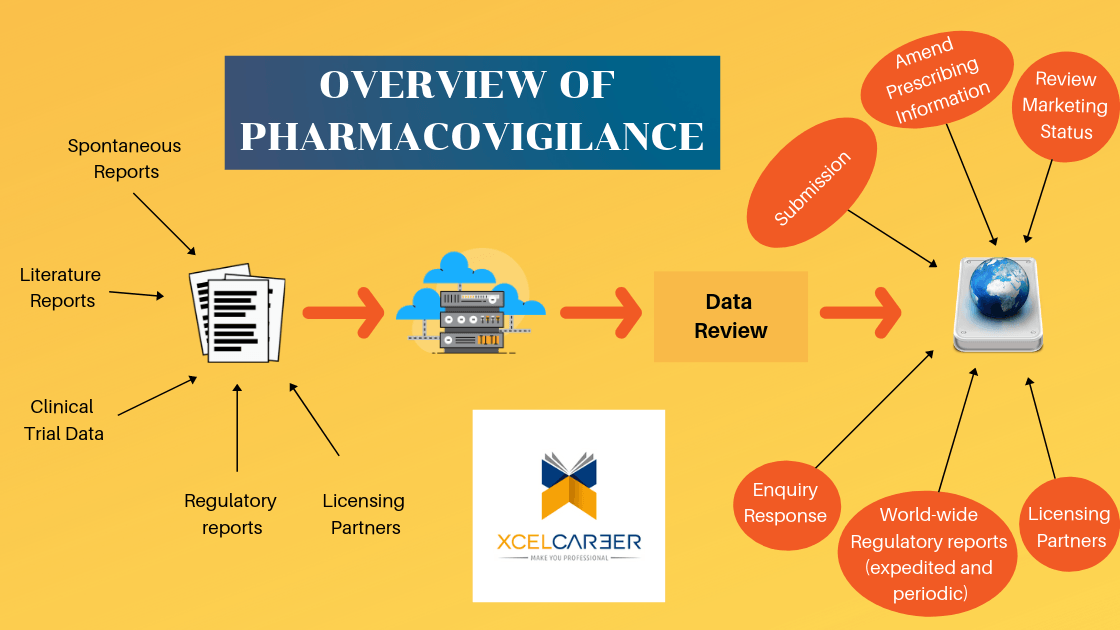
In the USA the total cost incurred due to medication errors or adverse drug reactions (ADRs) exceed 136 billion USD annually and hence, the regulatory agencies worldwide have developed stringent guidelines for drug approvals as well as for continuous monitoring of drugs in clinical practice.
The World Health Organization (WHO) has defined pharmacovigilance as the science and all the associated activities related to the detection, evaluation, understanding and prevention of ADRs or any other drug-related problems. ADRs (especially the serious drug reactions or SAEs), medication and prescription errors and poor drug qualities are the major concerns which are dealt with by pharmacovigilance systems. Thus, risk management, risk mitigation plans and discovery of safety signals from data mining of existing safety databases are immensely important activities during the development as well as post marketing phases in the life cycle of a drug. Pharmacovigilance is an important and integral part of clinical research and these days it is growing in many countries and many pharmacovigilance centers are working for drug safety monitoring in this global pitch.
A very important constituent of a compliant pharmacovigilance program is to maintain large safety databases. Such requirements of maintaining and analyzing extremely large quantities of data has pushed pharmaceutical companies to outsource pharmacovigilance data management and analysis to cost effective hubs like India, Philippines, China, to name a few major ones. It is pertinent to note that the outsourced pharmacovigilance activity to India is on a steep rise for last 5 years. With the heavy outsourcing comes the need of trained professionals, and so XCEL CAREER
Objective of Pharmacovigilance
- Proactive monitoring and reporting on the quality, safety and efficacy of drugs,
- Assessment of the risks and benefits of marketed medicines,
- Monitoring the impact of any corrective actions taken,
- Providing information to consumers, practitioners and regulators on the effective use of drugs,
- Designing programs and procedures for collecting and analysing reports from health care professionals (HCPs), patients, relatives, lawyers, journalists etc.
- Early detection of unknown/unexpected safety problems,
- Detection of increases in frequency of Adverse Drug Reactions (ADRs) to a drug, in patients/subjects treated with this medication,
- Identification of risk factors for ADRs,
- Risks Analysis & Mitigation,
- Benefit-Risk balance.
Pharmacovigilance Module overview
- Introduction to PV
- Process of PV
- The PV Jargons
- GVP
- Narrative Writing
- A. E. Reporting
- Pharmacovigilance in India
- Medical Coding

Career in Pharmacovigilance Jobs in India
You have varied jobs and totally different roles in regulative affairs; you’ll begin your career as a drug safety organizer, associate or scientist. With expertise, you’ll realize varied positions in acknowledged MNCs.

“SUCCESS BESEECHES PROCESS” – which includes LEARNING, ENHANCING SKILLS with POSITIVE ATTITUDE

 Pharmacovigilance Course
Pharmacovigilance Course