 Clinical Data Management Course
Clinical Data Management Course

When new drugs or devices are tested in humans, the data generated by and related to these trials is known as clinical data.
Definition of Clinical Data Management:
Clinical data management (CDM) is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. Clinical data management assures collection, integration and availability of data at appropriate quality and cost.
CLINICAL DATA MANAGEMENT is a process of ensuring that data collected during a clinical trial is…
- Accurate
- Complete
- Logical
- Consistent
Objectives of CLINICAL DATA MANAGEMENT:
To Ensure:
- That collected data is complete & accurate so that results are correct.
- That trial database is complete, accurate & a true representation of what took place in trial.
- That trial database is sufficiently clean to support statistical analysis & its subsequent presentation & interpretation.
Clinical Data Management Overview
- Clinical Trials and drug discovery introduction
- Principles of Clinical trials
- 21CFR part 11 introduction
- GCP (Good Clinical Practice Principles
- Clinical Data Management introduction
- Clinical Data Management phases
- Start-up Phase introduction. Conduct Phase introduction
- Discrepancy Management
- Data set validation review
- Offline Listing review
- All third party vendor reconciliation
- SAE reconciliation
- Study close-out phase introduction and activities
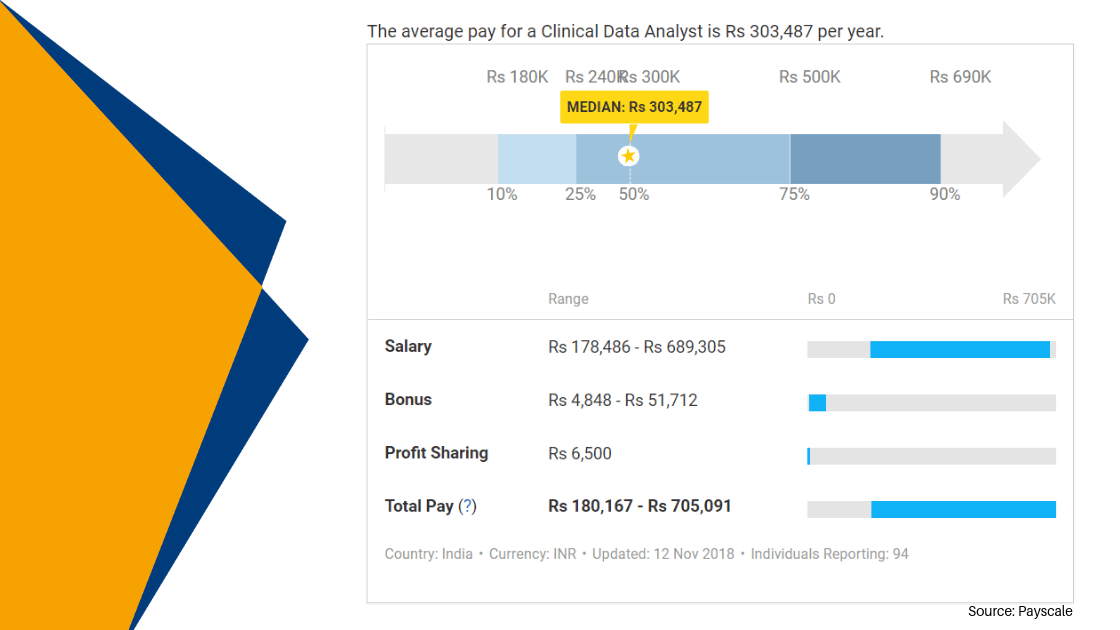
Clinical Data Management Jobs
Xcelcareer is one the best Clinical Data Management Institute in Hyderabad . We provide Advance clinical research training such as Clinical Data Management Training and Clinical Data Management Course in India, Hyderabad.

 Clinical Data Management Course
Clinical Data Management Course