 MEDICAL WRITING
MEDICAL WRITING
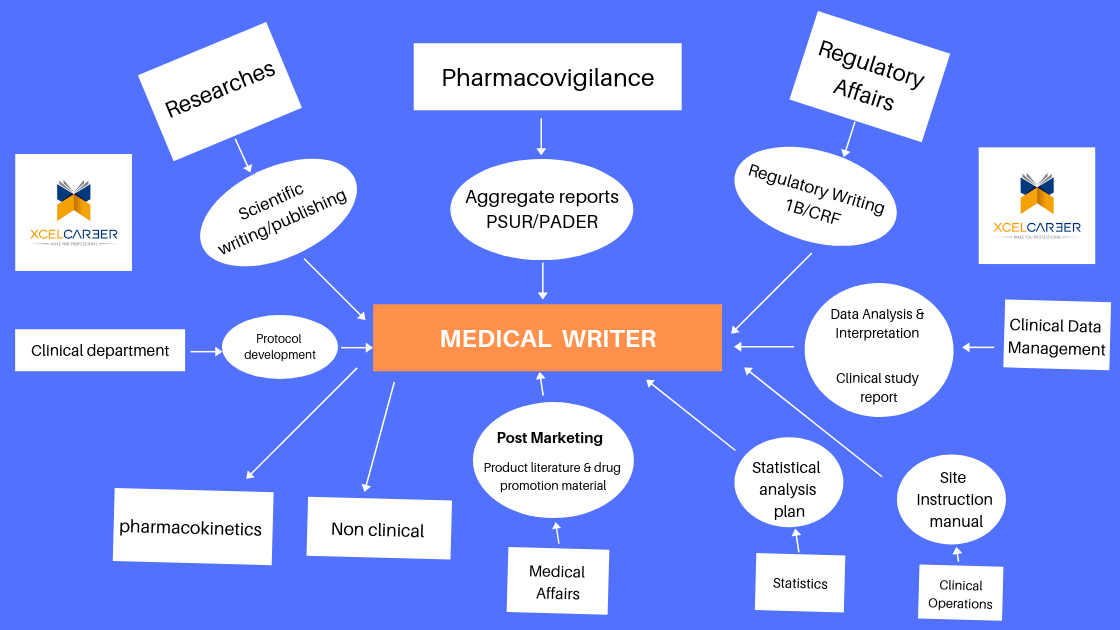
What is Medical Writing?
Medical writing can be of several types like research writing, product related promotional writing, educational materials for patients or researchers or physicians, manuscripts, abstracts, health-related news articles, regulatory, websites of healthcare or pharmaceutical companies and many others. These medical or scientific information documents should be written according to the type of target audiences like patients, doctors or physicians, general public or the regulatory officials. What makes medical writing a challenging job is the need of a clear thinking about the content and the respective audience
Definition of Medical writing
Medical writing is the activity of producing scientific documentation by a specialised writer. The medical writer typically is not one of the scientists or doctors who performed the research.
A medical writer, working with doctors, scientists, and other subject matter experts, creates documents that effectively and clearly describe research results, product use, and other medical information. The medical writer also ensures that their documents comply with regulatory, journal, or other guidelines in terms of content, format, and structure.
Medical writing as a function became established in the pharmaceutical world because the industry recognised it requires special skill to produce well-structured documents that present information clearly and concisely. A growing number of new drugs go through the increasingly complex process of clinical trials and regulatory procedures that lead to market approval. This drives a demand for well written, standards-compliant documents that medical and science professionals can easily and quickly read and understand.
Objectives of Medical Writing
Medical writing for the pharmaceutical industry can be classified as either regulatory medical writing or educational medical writing.
Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and biologics. Regulatory documents can be huge and are formulaic. They include clinical study protocols, clinical study reports, patient informed consent forms, investigator brochures and summary documents (e.g. in Common Technical Document [CTD] format) that summarise and discuss the data a company gathers in the course of developing a medical product.
Educational medical writing means writing documents about drugs, devices and biologics for general audiences, and for specific audiences such as health care professionals. These include sales literature for newly launched drugs, data presentations for medical conferences, medical journal articles for nurses, physicians and pharmacists, and programs and enduring materials for continuing education (CE) or continuing medical education (CME).
Other types of medical writing include journalism and marketing, both of which can have a medical writing focus.
Regardless of the type of medical writing, companies either assign it to an in-house writer or “outsource” it to a contract medical writer or medical writing service.
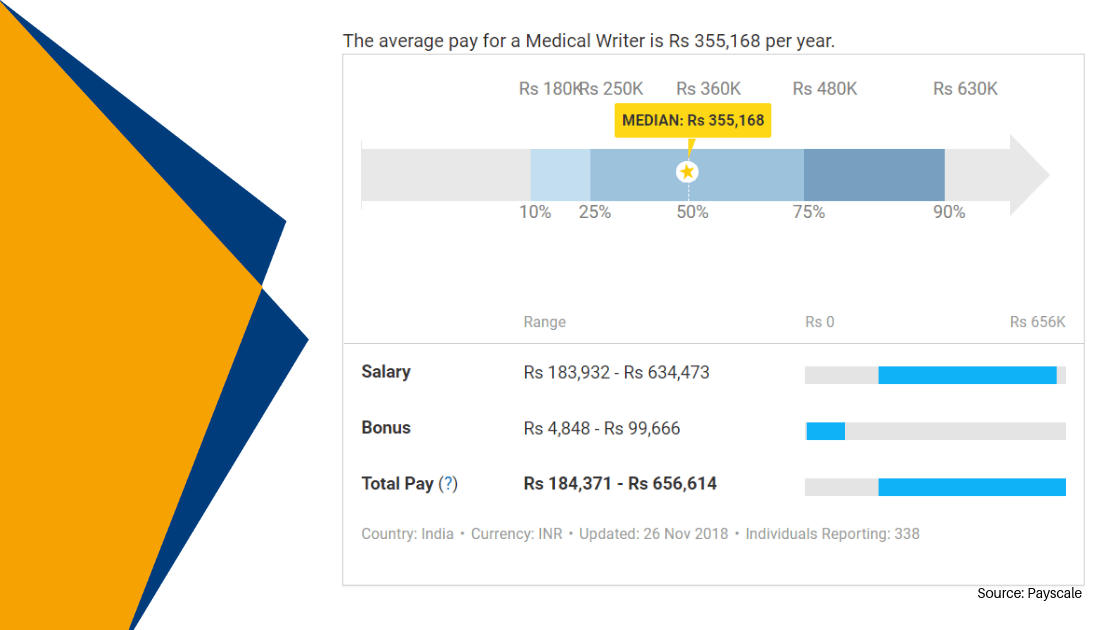

- Xcel career aim is to be the Best Educational Institute in the decade by accomplishing the employability skills of the future generations of Professionals by offering specialized training courses into various healthcare sectors such as Clinical Research, Medical Coding, and Pharma Health Management.
- “SUCCESS BESEECHES PROCESS” – which includes LEARNING, ENHANCING SKILLS with POSITIVE ATTITUDE

 MEDICAL WRITING
MEDICAL WRITING