Health and Healing
Good health is indeed a boon and requires constant monitoring by developing good habits and following a relaxed routine. But do we give priority to our health needs? What comes to our rescue when in trouble? The undisputed answer is “medicines”, which are just prescriptions away.
Now the question is what if we suffer from a disease which has no definite treatment or whose treatment is extremely expensive or the available drugs have poor efficacy and safety? Thus, arises a need for developing a new medicine which can protect us from such diseases, provide relief and that is pocket-friendly. This is achieved a specialized discipline called ‘CLINICAL RESEARCH’ which focuses on innovating, developing and turning new molecules into therapeutic drugs. This process of drug development is unique as it involves testing of new drugs on Human subjects before getting entry into the market.
Uncompromising Skills
How can we conduct experiments on human beings? Is it ethical to do so? How does law permit this? Do we require the consent of participating subjects? Does the medical fraternity allow such experiments? Are they scientifically acceptable? All these concerns pose a big challenge to medical science which has been addressed through an internationally accepted approach called ‘Clinical Trails’. It refers to a systematic assessment of the safety and efficacy of an investigational drug on human subjects on under controlled experimental subjects.
Pursuing a Career
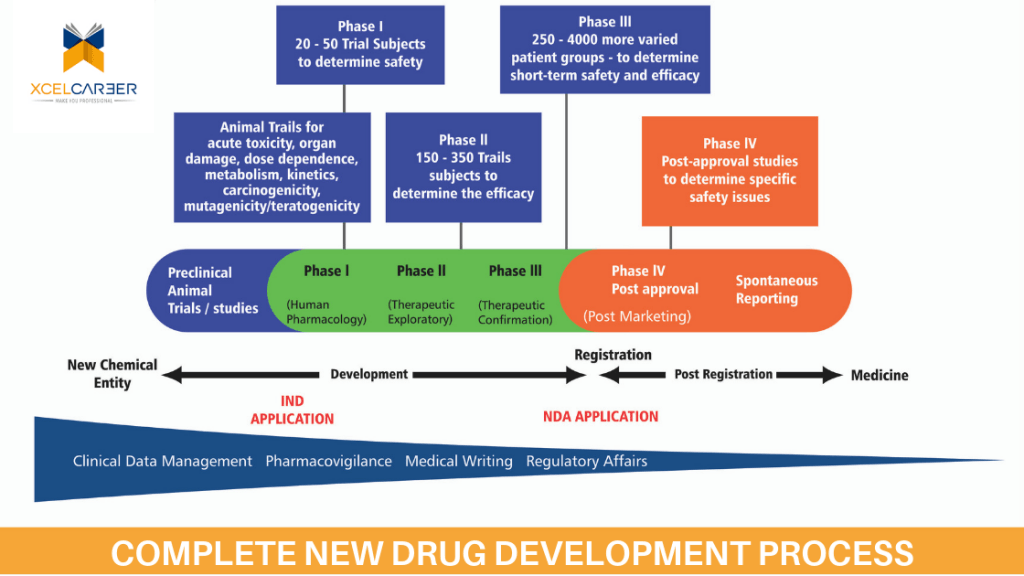
Clinical Research aims at verifying the results of animal studies in Human beings and establishes the therapeutic value of a new Chemical entity (NCE). This is done phases with an increasing number of participating subjects revealing the biological benefits of the test compounds. This involves a whole lot of planning, a large number of professional and patients. It is a fairly long process involving complex product designing elaborate pre-clinical testing, safe clinical evaluation, and stringent regulatory submissions and controlled marketing approvals. The same sequence applies to vaccines, medical devices, surgical procedures and biopharmaceutical product before their commercial launch. The whole gamut of activities takes around ten to twelve years and a huge investment of Billions of dollars since the success rate is one in a thousand or even less
Opportunities are plenty
The innovative Pharmaceutical companies generally outsourced the various trial-related activities to contract research organizations (C.R.O) that hold the experience and expertise to execute these studies and its back-hand processes. With the new amendments in the Indian drug regulation, Schedule –Y and others. The market share of Clinical trials and its outsourcing of backhand processes such as Pharmacovigilance, Clinical Data Management, Regulatory Affairs, Clinical SAS and Medical and Scientific writing expected to grow many folds from current base owing to speed, time and cost advantage which India offers then other developing countries.
The revenue generation will increase from to reach around $986.9 Million from $500 Million in 2017, by the end of 2023 at a CAGR of ~12.00%. This would require 50000 clinical research professionals trained in Good Clinical Practice (GCP) to handle Clinical Research outsourcing work be it Clinical trials, product development, process development, post-marketing surveillance, quality monitoring, and others. as per the Global standards. The opportunities are plenty but the takers are scanty.
A CRO provides support to the pharmaceutical, biotechnology, and medical device industries for specific services on a contract basis. The services may be clinical research, product development such as drug development or process development such as bioanalytical assay development. A CRO may also provide management services such as clinical trials management and Pharmacovigilance.
India is emerging as a top destination for contract research organizations for the following reasons
• India’s acceptance of International guidelines and intellectual property rights
• Presence of diverse types of climatic conditions thus allowing stability studies to be performed with ease in one destination
• Educated and accessible human resource in India
• Presence of diverse ethnic pool thus enabling diverse sample for clinical trials
• Low operational cost due to cheap human resource
• Availability of the largest pool of patients and large hospitals
The other factors favoring the market are efforts by the regulatory authorities such as Director Controller General of India (DCGI), Indian Council of Medical Research (ICMR), Directorate General of Foreign Trade (DGFT), Department of Biotechnology (DBT) to create an amenable climate for research in India.
Growth of PV in India
Pharmacovigilance (PV) has emerged as a globally recognized process of evaluating the safety profile of products and take risk minimization measures, right from clinical development until the product life cycle in the market. It involves multiple stakeholders including the pharmaceutical companies who develop and manufacture the drugs, regulatory authorities who ensure that the market is regulated, patients taking the drugs and physicians and other healthcare professional involved in patient care.
PV has seen tremendous growth in the last decade with job opportunities rising at a very rapid rate. The companies are required to monitor the drug under current and emerging regulations around the globe. While many developed countries like EU and USA have already established regulations, many developing countries like India and China are working on coming up with new regulations around PV which will facilitate companies in these markets to increase focus on PV activities.
The companies are becoming more cognizant about the collection of information from multiple sources including patients and prescribers and also through their marketing programs which is a big source of adverse events. There is an increase of the adverse events reports by at least 10-15% majorly due to increasing awareness and number of submissions worldwide. There is an increased focus on PV related activities and although this is an additional cost to the company, they are investing in this cost to monitor the safety profile of their products and avoid higher impact like recall of products from the market which brings down the value and trust of the companies.
With new regulations in the form of E2B R3 and Centralized EudraVigilance download of ICSRs for post-marketing cases has further contributed to the increase in the number of ICSRs reported to a company.
The increasing PV activities have a direct impact on the growth of outsourcing of these activities to Indian service providers for cost-effectiveness and resource efficiency. The increasing adverse event collection and reporting will contribute to a significant increase in the PV market in these countries. The projected yearly increase of 10-15% in the number of adverse events reported, definitely shows a proportional growth opportunity for PV market to facilitate the monitoring, analyzing and reporting this data.
Though there is a focus on digitalization and automation of certain aspects of ICSR management and other activities, there is still an increasing demand of jobs in PV due to increased regulations I around the globe around PV and increased complexity of the medicines.
The new personalized medicines need specific attention and monitoring which cannot be automated with current development in automation in this space. The advancement in targeted therapies will further drive the demand for pharmacovigilance services in the near future.
Therefore, there is an expected increase in PV Jobs in India for more than 10% yearly to cater to the future needs of PV and its increasing demand and complexities.
The Face of the Future
As per the market reports, nearly 50000 trained manpower is required for testing these experimental drugs and related Pharmacovigilance process, etc. but the country is facing a crunch of trained manpower. Short term in-house training programs are conducted by managers resulting in the passive acquisition of knowledge that is generally job specific and does not fulfill the multitasking needs of the industry.
All these efforts go for a toss when the trained candidate decides to join the competitive company offering a higher salary. This not only creates an unpleasant and undesirable situation but also hampers the on-going projects. Thus, there is an acute need for trained clinical research professional for conducting studies as per international standards and guidelines.
Several Clinical Research academies are chipping into bridge the gap between current demand and supply. Here the concern is that the curriculum must be based on industry needs both in terms of technical knowledge as well as the soft skills that are essential as a team player.
These academies should have established a link with C.R.O’s / Hospitals /IT’es providing them an opportunity to participate in on-going studies, in India or abroad and its backhand processes with their freshly acquired knowledge.
A well-designed program will prepare the candidates for a global career in the clinical research industry with a thorough understanding of the national and international guidelines on patient safety, ethical issues, standard operating procedures and regulatory environment in various regions of the world.
XCELCAREER has made its presence felt starting a 2 to 3 months ISEP Certification in Clinical Research in association with the industry, who have reviewed and given their feedback which has helped in designing the curriculum to meet the precise requirements of the industry.
Deciphering Global Education
Clinical Research being a multidisciplinary science offers a good platform to Pharmacy, life science, Biotechnology, and medical graduates to shape up their career in this global industry. Depending upon the orientation and interest one can enter this field as an investigator, clinical research associate, monitor, data safety manager, quality assurance executive, regulatory manager, medical writer, data manager, project manager, Pharmacovigilance executive, and business development executive and up the ladder to reach the level of being the global head. Every member of the study team as a significant role to play for the success of the trail.
Various ethical guidelines and regulatory agencies not impart projections to the participating subjects but also help in generating data to draw meaningful conclusions. One successful drug changes the face of medical treatment as well as society.
No wonder, the challenges, but the returns are bigger.